Your Periodic table groups 1 8 images are available in this site. Periodic table groups 1 8 are a topic that is being searched for and liked by netizens now. You can Download the Periodic table groups 1 8 files here. Download all free photos.
If you’re looking for periodic table groups 1 8 pictures information linked to the periodic table groups 1 8 interest, you have visit the right site. Our website frequently provides you with hints for refferencing the maximum quality video and picture content, please kindly search and find more enlightening video content and graphics that match your interests.
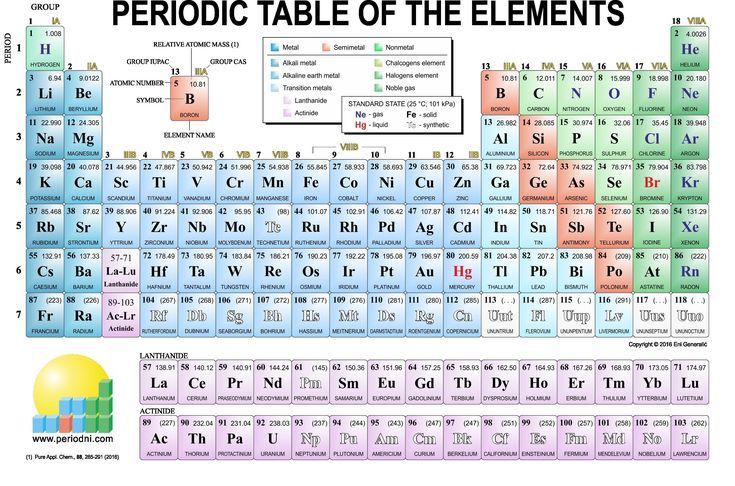
Periodic Table Groups 1 8. The group number is an identifier used to describe the column of the standard periodic table in which the element appears. The elements in each group have the same number of valence electrons. These are labelled from 1 to 18 under current iupac numenclature. These are the horizontal rows that show the number of shells of electrons an atom has
 Pin de Leonardo Sotillo en Tabla periodica actual Tabla From pinterest.com
Pin de Leonardo Sotillo en Tabla periodica actual Tabla From pinterest.com
Gold task dimitri mendeleev created the periodic table we use today in 1869. • he grouped elements according to their atomic mass, and as he did, he found that the groups had similar chemical properties. Columns of the periodic table typically mark groups or families. Alkali metals, or lithium family; Placed in the vertical column on the far right of the periodic table. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game!
See about the periodic table for information on how group can be used to characterize an element.
Terms in this set (44) 1. The older iupac system used roman numerals together with letters to distinguish between the left (a) and right (b) side of the periodic table. Elements in these groups are collectively known as main group or representative elements. Periodic table groups vs periods; These are the horizontal rows that show the number of shells of electrons an atom has • blank spaces were left open to add the new elements he predicted would occur.
 Source: pinterest.com
Source: pinterest.com
Grouped elements behave chemically in similar ways. Make a poster to show at least 6 of the elements from these three groups and what we use them for. The group number is an identifier used to describe the column of the standard periodic table in which the element appears. Sulphur, which can form two single bonds to itself, comes in various ring sizes up to s 20, s 8 being the most stable. They exist as single atoms.
 Source: pinterest.com
Source: pinterest.com
A group is also known as a family of atoms in which elements are arranged within each group of the periodic table. The columns represent the groups. Write/draw your findings on the blank page provided. Find out about mendeleev and how he created the pattern of the periodic table. The periodic table of the chemical elements.
 Source: pinterest.com
Source: pinterest.com
Groups and periods the periodic table is a way of arranging the elements so patterns in their properties and reactions can be identified and explained. Groups groups run vertically in the periodic table. A period is a horizontal row on the periodic table. They include lithium (li), sodium (na) and potassium (k). Periodic table of element groups.
 Source: pinterest.com
Source: pinterest.com
Periodic table groups 3 12; Group 7 and group 0 of the periodic table. And so let�s move on to the concept of periods. Only the top portion of the periodic table is shown above (full version)a very brief introduction to the periodic table (part of which is shown above). The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because.
 Source: pinterest.com
Source: pinterest.com
Summary of the structure of the periodic table. Periodic table groups and families; As a result, elements in the same group often display similar properties and reactivity. Grouped elements behave chemically in similar ways. Periodic table groups 1 8;
 Source: pinterest.com
Source: pinterest.com
Click on the element symbol in the table for further information. Metals are very reactive with chemical reactivity increasing down the group. Periodic table of element groups. There are total 18 numbered groups in the modern periodic table, however, the “f” block columns between the group 2 and 3 are not numbered. Placed in the vertical column on the far right of the periodic table.
 Source: pinterest.com
Source: pinterest.com
These are the horizontal rows that show the number of shells of electrons an atom has And so let�s move on to the concept of periods. They include lithium (li), sodium (na) and potassium (k). They are highly reactive and do not occur freely in nature. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
 Source: pinterest.com
Source: pinterest.com
Groups and periods the periodic table is a way of arranging the elements so patterns in their properties and reactions can be identified and explained. Metals are very reactive with chemical reactivity increasing down the group. The other ten groups are transition metals: Only the top portion of the periodic table is shown above (full version)a very brief introduction to the periodic table (part of which is shown above). Groups are numbered from 1 to 18.
 Source: pinterest.com
Source: pinterest.com
Groups 1, 2 and 13 to 18 constitute the main group. There are total 18 numbered groups in the modern periodic table, however, the “f” block columns between the group 2 and 3 are not numbered. Elements in these groups are collectively known as main group or representative elements. They are highly reactive and do not occur freely in nature. Grouped elements behave chemically in similar ways.
 Source: pinterest.com
Source: pinterest.com
Periodic table of element groups. Write/draw your findings on the blank page provided. These are the horizontal rows that show the number of shells of electrons an atom has Click on the element symbol in the table for further information. Periodic table groups vs periods;
 Source: pinterest.com
Source: pinterest.com
Groups are numbered from 1 to 18. As a result, elements in the same group often display similar properties and reactivity. The elements in group 0 are called the noble gases. And so let�s move on to the concept of periods. And so, if i look at period 1, and i just move across my periodic table, hydrogen is in the first period and so is helium.
 Source: pinterest.com
Source: pinterest.com
These groups gain valence electrons to make a compound. Click on the element symbol in the table for further information. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Periodic table groups are columns of elements found in the modern periodic table. • he grouped elements according to their atomic mass, and as he did, he found that the groups had similar chemical properties.
 Source: pinterest.com
Source: pinterest.com
Earlier labelling schemes (trivial group names) for historical reasons some groups have special names. Alkali metals, or lithium family; The periodic table of the chemical elements. Write/draw your findings on the blank page provided. The columns represent the groups.
 Source: pinterest.com
Source: pinterest.com
The elements in each group have the same number of valence electrons. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Write/draw your findings on the blank page provided. Group 7 and group 0 of the periodic table. The periodic table • in 1869,dmitri ivanovitch mendeléev created the first accepted version of the periodic table.
 Source: pinterest.com
Source: pinterest.com
Sulphur, which can form two single bonds to itself, comes in various ring sizes up to s 20, s 8 being the most stable. Find out about mendeleev and how he created the pattern of the periodic table. Check out this free, online educational resource. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because. The elements displayed in each periodic table group are either gas, liquid or solid at room temprature and are classified in groups as:
 Source: pinterest.com
Source: pinterest.com
Check out this free, online educational resource. See about the periodic table for information on how group can be used to characterize an element. Recognizing families on the periodic table. They exist as single atoms. Periodic table groups vs periods;
 Source: pinterest.com
Source: pinterest.com
Elements in the same groups have the same number of valence electrons in the outer energy level. Sulphur, which can form two single bonds to itself, comes in various ring sizes up to s 20, s 8 being the most stable. They include lithium (li), sodium (na) and potassium (k). The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because. Periodic table groups 3 12;
 Source: pinterest.com
Source: pinterest.com
The older iupac system used roman numerals together with letters to distinguish between the left (a) and right (b) side of the periodic table. Gold task dimitri mendeleev created the periodic table we use today in 1869. Summary of the structure of the periodic table. The other ten groups are transition metals: Metals are very reactive with chemical reactivity increasing down the group.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title periodic table groups 1 8 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






