Your Electron configuration of neon ion images are available in this site. Electron configuration of neon ion are a topic that is being searched for and liked by netizens today. You can Find and Download the Electron configuration of neon ion files here. Get all free images.
If you’re looking for electron configuration of neon ion images information related to the electron configuration of neon ion keyword, you have pay a visit to the right blog. Our website always provides you with hints for viewing the highest quality video and image content, please kindly hunt and locate more informative video content and graphics that match your interests.
Electron Configuration Of Neon Ion. Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital. It is a noble gas. The same as that of a neon atom The electron configuration is the same as for neon and the number of nonvalence electrons is 2.
 Pin on Anthony Burrill From pinterest.com
Pin on Anthony Burrill From pinterest.com
Atoms and atomic structure chemistry noble gases elements and compounds science experiments. Therefore, the abbreviated electron configuration of sodium is [ne]3s 1 (the electron configuration of neon is 1s 2 2s 2 2p 6, which can be abbreviated to [he]2s 2 2p 6). Just replace this portion of zinc�s electron notation with argon�s chemical symbol in brackets ([ar].) so, zinc�s electron configuration written in shorthand is [ar]4s 2 3d 10. Oxygen in a neutral state would have 8 total electrons (6 valence). Determining the valency of an element. The same for na+ which has lost an electron and also is na+2,8.
Zinc�s full electron configuration is:
The same for na+ which has lost an electron and also is na+2,8. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. The electronic configuration of an atom gives us the idea of how the electrons of the atom are distributed in different orbits and orbitals in the increasing order of the energy. The electronic configuration of oxygen is 1s^2 2s^2 2p^4 the charge of oxide ion is —2, which means an oxygen atom gained 2 extra electrons. For example, consider the compound formed from aluminum and oxygen. Therefore the ne electron configuration will be 1s22s22p6.
 Source: in.pinterest.com
Source: in.pinterest.com
The same principles can be applied to many other cases. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. 1s2 2s2 2p6 3s2 3p5: There are 118 elements in the periodic table. Because it has one unpaired electron, it is paramagnetic.
 Source: pinterest.com
Source: pinterest.com
The b atom has 2s 2 2p 1 as the electron configuration. We can abbreviate the electron configuration by indicating the innermost electrons with the symbol of the preceding noble gas. Therefore, br has 1 unpaired electron. The same as that of a neon atom Electron configuration chart for all elements in the periodic table.
 Source: pinterest.com
Source: pinterest.com
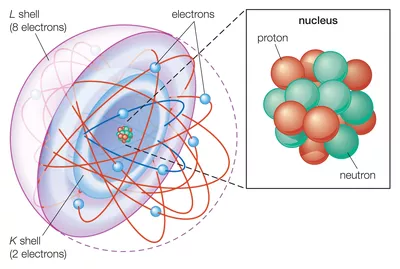
Neon is the tenth element with a total of 10 electrons. Neon has the electron configuration ne2,8. The preceding noble gas with an atomic number less than sodium is neon, ne. So,this two extra electrons will be attached the. Look at a perodic table and find neon (it�s on the far right, second row).
 Source: pinterest.com
Source: pinterest.com
The br atom has 4s 2 3d 10 4p 5 as the electron configuration. The remaining six electrons will go in the 2p orbital. The electron configuration of a fluoride ion, f⁻, is _____ a. Therefore the ne electron configuration will be 1s22s22p6. Therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6.
 Source:
Source:
Once again, the electron configuration is the same as in the previous examples and the number of. 1s2 2s2 2p6 3s2 3p5: 1s2 2s2 2p6 3s2 3p4: Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital. An atom of neon in the gas phase, for example, gives off energy when it gains an electron to form an ion of neon.
 Source: pinterest.com
Source: pinterest.com
Neon is the tenth element with a total of 10 electrons. We rewrite the electron configuration: Recall, the electron configuration for na is: Look at a perodic table and find neon (it�s on the far right, second row). Then move two elements to the left.
 Source: pinterest.com
Source: pinterest.com
Neon has the electron configuration ne2,8. For example, the electron configuration of the neon atom (ne) is 1s 2 2s 2 2p 6. Neon has the electron configuration ne2,8. When an electron is added to a neutral atom, energy is released. Electron configuration chart for all elements in the periodic table.
 Source: pinterest.com
Source: pinterest.com
1s 2 2s 2 2p 6 given : Oxygen in a neutral state would have 8 total electrons (6 valence). Thus, you should write the electron configuration for 10 electrons. The electron configuration is the same as for neon and the number of nonvalence electrons is 2. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
 Source: pinterest.com
Source: pinterest.com
In writing the electron configuration for neon the first two electrons will go in the 1s orbital. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. So,this two extra electrons will be attached the. Because it has no unpaired electrons, it is. For example, consider the compound formed from aluminum and oxygen.
 Source: in.pinterest.com
Source: in.pinterest.com
Oxygen in a neutral state would have 8 total electrons (6 valence). Neon is a chemical element with the symbol ne and atomic number 10. The same principles can be applied to many other cases. Recall, the electron configuration for na is: For example, consider the compound formed from aluminum and oxygen.
 Source: pinterest.com
Source: pinterest.com
Mathematically, configurations are described by slater determinants or configuration state func Recall, the electron configuration for na is: However, there can be a few positive or negative charge ions with the same number of electrons/electron configuration as neon. The remaining six electrons will go in the 2p orbital. We can abbreviate the electron configuration by indicating the innermost electrons with the symbol of the preceding noble gas.
 Source: pinterest.com
Source: pinterest.com
Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation. Because it has no unpaired electrons, it is. We can abbreviate the electron configuration by indicating the innermost electrons with the symbol of the preceding noble gas. Neon has the electron configuration ne2,8. The electron configuration is the same as for neon and the number of nonvalence electrons is 2.
 Source: pinterest.com
Source: pinterest.com
According to the laws of quantum mechanics, a certain energy is associated with each. An atom of neon in the gas phase, for example, gives off energy when it gains an electron to form an ion of neon. Neon is in the second period and the group 18 of the periodic table. The remaining six electrons will go in the 2p orbital. Elemental fluorine has an electron configuration of 1s22s22p5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion.
 Source: pinterest.com
Source: pinterest.com
Therefore, br has 1 unpaired electron. Therefore, the abbreviated electron configuration of sodium is [ne]3s 1 (the electron configuration of neon is 1s 2 2s 2 2p 6, which can be abbreviated to [he]2s 2 2p 6). Therefore the ne electron configuration will be 1s 2 2s 2 2p 6. We can abbreviate the electron configuration by indicating the innermost electrons with the symbol of the preceding noble gas. To achieve the neon configuration, aluminum must lose three electrons, forming the al3+ ion.
 Source: pinterest.com
Source: pinterest.com
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Thus it gains an electron when forming the fluoride ion, and becomes isoelectronic to neon. Mathematically, configurations are described by slater determinants or configuration state func Zinc�s full electron configuration is:
 Source: pinterest.com
Source: pinterest.com
Once again, the electron configuration is the same as in the previous examples and the number of. 1s2 2s2 2p6 3s2 3p6 4s2 The br atom has 4s 2 3d 10 4p 5 as the electron configuration. Because it has one unpaired electron, it is paramagnetic. 1s2 2s2 2p6 3s2 3p6:
 Source: pinterest.com
Source: pinterest.com
The electron configuration of a fluoride ion, f⁻, is _____ a. To achieve the neon configuration, aluminum must lose three electrons, forming the al3+ ion. 1s2 2s2 2p6 3s2 3p6 4s1: This would add 2 electrons to its normal configuration making the new configuration: Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital.
 Source: pinterest.com
Source: pinterest.com
In other words 2 electrons in the inner shell and 8 in the outer full shell. The remaining six electrons will go in the 2p orbital. Aluminum has the electron configuration [ne]3s23p1. Neon is the tenth element with a total of 10 electrons. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electron configuration of neon ion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






